The reaction mechanism of hydroxyethylphosphonate dioxygenase: a QM/MM study†
Abstract
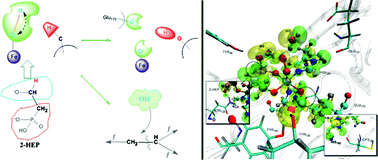
By employing ab initio quantum mechanical/molecular mechanical (QM/MM) and molecular dynamics (MD) simulations, we have provided further evidence against the previously proposed hydroperoxylation or ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group serves as a general base. The reaction directions seem to be tunable and show significant environment dependence. This mechanism can provide a comprehensive interpretation for the seemingly contradicting experimental evidences and provide insight into the development of biochemistry and material sciences.
O group serves as a general base. The reaction directions seem to be tunable and show significant environment dependence. This mechanism can provide a comprehensive interpretation for the seemingly contradicting experimental evidences and provide insight into the development of biochemistry and material sciences.

 Please wait while we load your content...
Please wait while we load your content...