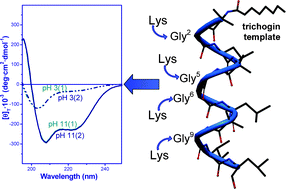
Trichogin GA IV, isolated from the fungus Trichoderma longibrachiatum, is the prototype of lipopeptaibols, the sub-class of short-length peptaibiotics exhibiting membrane-modifying properties. This peptaibol is predominantly folded in a mixed 310-/α- helical conformation with a clear, albeit modest, amphiphilic character, which is likely to be responsible for its capability to perturb bacterial membranes and to induce cell death. In previous papers, we reported on the interesting biological properties of trichogin GA IV, namely its good activity against Gram positive bacteria, in particular methicillin-resistant S. aureus strains, its stability towards proteolytic degradation, and its low hemolytic activity. Aiming at broadening the antimicrobial activity spectrum by increasing the peptide helical amphiphilicity, in this work we synthesized, by solution and solid-phase methodologies, purified and fully characterized a set of trichogin GA IV analogs in which the four Gly residues at positions 2, 5, 6, 9, lying in the poorly hydrophilic face of the helical structure, are substituted by one (position 2, 5, 6 or 9), two (positions 5 and 6), three (positions 2, 5, and 9), and four (positions 2, 5, 6, and 9) Lys residues. The conformational preferences of the Lys-containing analogs were assessed by FT-IR absorption, CD and 2D-NMR techniques in aqueous, organic, and membrane-mimetic environments. Interestingly, it turns out that the presence of charged residues induces a transition of the helical conformation adopted by the peptaibols (from 310- to α-helix) as a function of pH in a reversible process. The role played in the analogs by the markedly increased amphiphilicity was further tested by fluorescence leakage experiments in model membranes, protease resistance, antibacterial and antifungal activities, cytotoxicity, and hemolysis. Taken together, our biological results provide evidence that some of the least substituted among these analogs are good candidates for the development of new membrane-active antimicrobial agents.

 Please wait while we load your content...
Please wait while we load your content...