Silver-catalysed intramolecular cyclisation of 2-alkynylacetophenones and 3-acetyl-2-alkynylpyridines in the presence of ammonia
Abstract
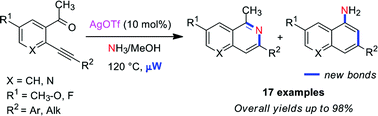
Silver-catalysed/microwave-assisted domino reactions of 2-alkynyl-acetophenones and 3-acetyl-2-alkynylpyridines in the presence of

 Please wait while we load your content...
Please wait while we load your content...