Reinvestigation of the C5-acetamide sialic acid donor for α-selective sialylation: practical procedure under microfluidic conditions†
Abstract
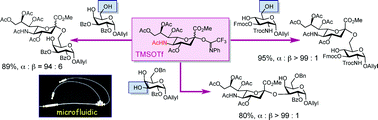
Despite the previous literature describing the “low-to-modest” efficiency, the readily available C5-acetamide donor was reinvestigated for its use in α-sialylation under microfluidic conditions. The N-phenyltrifluoroacetimidate donor was efficiently mixed with an appropriate amount of

 Please wait while we load your content...
Please wait while we load your content...