The photo-dehydro-Diels–Alder (PDDA) reaction
Abstract
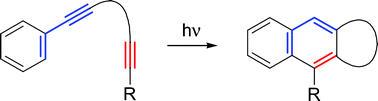
The photo-dehydro-Diels–Alder (PDDA) reaction is a valuable extension of the classical Diels–Alder (DA) reaction. The PDDA reaction differs from the DA reaction by the replacement of one of the C–C-double bonds of the diene moiety by a C–C triple bond and by the photochemical triggering of the reaction. This entails that, in contrast to the DA reaction, the PDDA reaction proceeds according to a multistage mechanism with biradicals and cycloallenes as intermediates. The PDDA reaction provides access to a considerable variety of compound classes. For example, 1-phenylnaphthlenes, 1,1′-binaphthyls, N-heterocyclic

 Please wait while we load your content...
Please wait while we load your content...