Total synthesis of clavaminol A, C and H†
Abstract
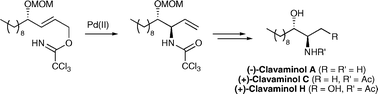
The first total synthesis of clavaminol A and C, (2R,3S)-2-amino-3-alkanols from the Mediterranean ascidian Clavelina phlegraea has been achieved in 29% overall yield. The key step involved a palladium(II)-catalysed directed Overman rearrangement to create the C–N bond and install the erythro configuration while a one-pot, tributyltin hydride-mediated reduction allowed simultaneous formation of the methyl side-chain and N-acetyl group. Similarly, the first total synthesis of clavaminol H was completed in 48% overall yield using an approach that also provided the cytotoxic des-acetyl analogue.

 Please wait while we load your content...
Please wait while we load your content...