3-Methoxalylchromones – versatile reagents for the regioselective synthesis of functionalized 2,4′-dihydroxybenzophenones, potential UV-filters†
Abstract
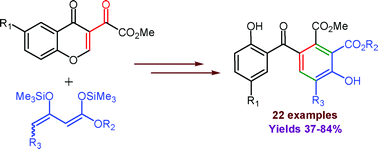
The reaction of 1,3-bis-silyl enol ethers with 3-methoxalylchromones affords a great variety of functionalised 2,4′-dihydroxybenzophenones. These products are formed by a domino Michael/retro-Michael/Mukaiyama-

 Please wait while we load your content...
Please wait while we load your content...