A general phosphoric acid-catalyzed desymmetrization of meso-aziridines with silylated selenium nucleophiles†
Abstract
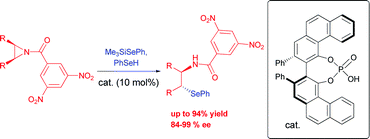
The first example of meso-aziridine desymmetrization with selenium nucleophiles is reported. The reaction, promoted by VAPOL-hydrogen phosphate using

 Please wait while we load your content...
Please wait while we load your content...