A new general approach for the stereocontrolled synthesis of functionalised γ- and δ-lactams†
Abstract
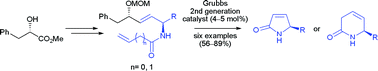
A new flexible approach for the stereoselective synthesis of substituted 1H-pyrrol-2(5H)-ones and 3,6-dihydro-1H-pyridin-2-ones has been developed. The general strategy employed the stereoselective reduction of a series of α,β-unsaturated
 Please wait while we load your content...
Please wait while we load your content...