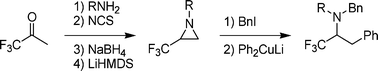
An efficient and straightforward approach towards the synthesis of 1-alkyl-2-(trifluoromethyl)aziridines starting from 1,1,1-trifluoroacetoneviaimination, α-chlorination, hydride reduction and ring closure was developed. In addition, novel primary β-iodo amines were obtained by regioselective ring opening of these 2-(trifluoromethyl)aziridines using alkyl iodides, and their synthetic potential was demonstrated by converting them into novel α-CF3-β-phenylethylamines upon treatment with lithium diphenylcuprate.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...