FeCl3-Mediated synthesis of polysubstituted tetrahydroquinolines via domino Mannich/Friedel–Crafts reactions of aldehydes and amines†
Abstract
A useful method to construct highly substituted tetrahydroquinolines has been developed through an iron(III) chloride-mediated domino Mannich and intramolecular Friedel–Crafts
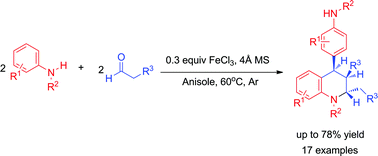
 Please wait while we load your content...
Please wait while we load your content...