A straightforward synthetic access to symmetrical glycosyl disulfides and biological evaluation thereof†
Abstract
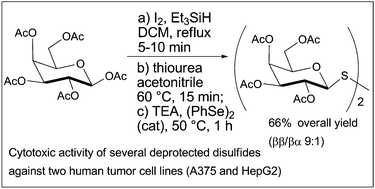
Symmetrical glycosyl disulfides can be prepared within a few hours from per-O-acetylated precursors via a sequential approach entailing short reactions and no

 Please wait while we load your content...
Please wait while we load your content...