A novel strategy of chemical modification for rate enhancement of 10–23 DNAzyme: a combination of A9 position and 8-aza-7-deaza-2′-deoxyadenosine analogs†
Abstract
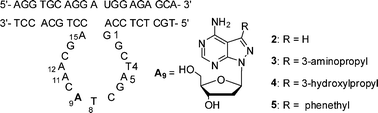
With the help of a divalent-metal ion, 10–23 DNAzyme cleaves RNA. Chemical modification of its catalytic loop to make a more efficient enzyme has been a challenge. Our strategy started from its five 2′-deoxyadenosine residues (A5, A9, A11, A12, and A15) in the loop based on the capability of the N7 atom to form hydrogen bonds in tertiary structures. 8-Aza-7-deaza-2′-deoxyadenosine and its analogs with 7-substituents (3-aminopropyl, 3-hydroxylpropyl, or phenethyl) were each used to replace five dA residues, respectively, and their effect on cleavage rate were evaluated under single-turnover conditions. The results indicated that the N7 atom of five dA residues were necessary for catalytic activity, and the N8 atom and 7-substituents were detrimental to the catalytic behavior of 10–23 DNAzyme, except that all these modifications at A9 were favourable for the activity. Especially, DZ-3–9 with 7-(3-aminopropyl)-8-aza-7-deaza-2′-deoxyadenosine (3) at A9 position gave a 12- fold increase of kobs, compared to the corresponding parent 10–23 DNAzyme. DZ-3–9 was supposed to catalyze the cleavage reaction with the same mechanism as 10–23 DNAzyme based on their very similar pH-dependent and divalent metal ions-dependent cleavage patterns. Introduction of functional groups at A9 position was demonstrated to be a successful and feasible approach for more efficient 10–23 DNAzyme analogs.

 Please wait while we load your content...
Please wait while we load your content...