Neutral species from “non-protic” N-heterocyclic ionic liquids†
Abstract
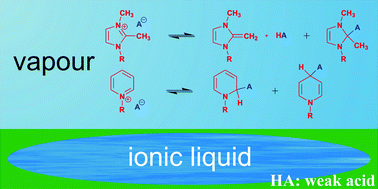
Possible isomerisation of 1,2,3-trialkylimidazolium and 1-alkylpyridinium ion pairs by proton transfer and by the nucleophilic addition of the anion to the cation have been investigated at the B3LYP/6-31+G* and B3LYP/6-311+G** levels of density functional theory. The deprotonation energies of 1,2,3-trialkylimidazolium and 1-alkylpyridinium cations to diaza-pentafulvene and pyridinium-ylide, respectively, were only slightly larger than that of 1,3-dialkylimidazolium salts yielding N-heterocyclic carbenes. Accordingly, in the case of 1,2,3-dialkylimidazolium salt ion pairs the stability of the H-bonded complex between the fulvene and the corresponding acid can be comparable to that of the ion pair in the presence of sufficiently basic anions, such as acetate. In the case of the pyridinium salts the nucleophilicity of the cation dominates over the acidity, and the formation of 1,2- or 1,4-dihydropyridine derivatives is preferred over proton transfer.

 Please wait while we load your content...
Please wait while we load your content...