Fluorine in medicinal chemistry: β-fluorination of peripheral pyrrolidines attached to acridineligands affects their interactions with G-quadruplex DNA†‡
Abstract
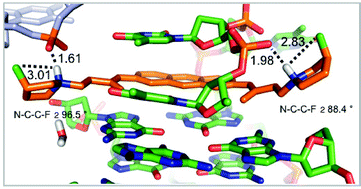
Comparative X-ray structure studies reveal that C–F bond incorporation into the peripheral pyrrolidine moieties of the G-quadruplex DNA binding
 Please wait while we load your content...
Please wait while we load your content...