Synthesis and evaluation of 5-lipoxygenase translocation inhibitors from acylnitroso hetero-Diels–Alder cycloadducts†
Abstract
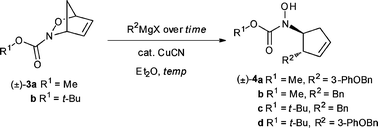
Acylnitroso cycloadducts have proven to be valuable intermediates in the syntheses of a plethora of biologically active molecules. Recently, organometallic reagents were shown to open bicyclic acylnitroso cycloadducts and, more interestingly, the prospect of highly regioselective openings was raised. This transformation was employed in the synthesis of a compound with excellent inhibitory activity against 5-lipoxygenase ((±)-4a, IC50 51 nM), an important mediator of inflammation intimately involved in a number of disease states including asthma and cancer. Optimization of the copper-mediated organometallic ring opening reaction was accomplished allowing the further exploration of the biological activity. Synthesis of a number of derivatives with varying affinity for metal binding as well as pendant groups in a range of sizes was accomplished. Analogues were tested in a whole cell assay which revealed a subset of the compounds to be

 Please wait while we load your content...
Please wait while we load your content...