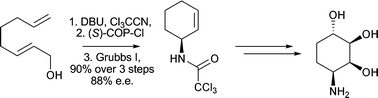
The stereoselective synthesis of a series of di- and tri-hydroxylated aminocyclohexane derivatives has been developed. A one-pot, two step tandem process involving an Overman rearrangement and a ring closing metathesis reaction has been utilised for the asymmetric synthesis of (1S)-1-(2′,2′,2′-trichloromethylcarbonylamino)cyclohexa-2-ene. Oxidation of this cyclohexene derivative was then studied leading to the preparation of two diol analogues in excellent stereoselectivity. (1S)-1-(2′,2′,2′-trichloromethylcarbonylamino)cyclohexa-2-ene was then converted to a novel allylic alcoholvia a 4,5-dihydro-1,3-oxazole. Functionalisation of this allylic alcohol by Upjohn dihydroxylation conditions or by a directed epoxidation/hydrolysis sequence of reactions allowed the synthesis of two dihydroconduramines in excellent stereoselectivity. The stereochemical assignment of all compounds prepared was confirmed by NOE experiments or X-ray structure determination.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...