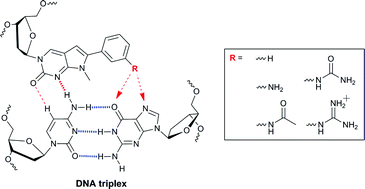
CG base pair recognition within DNA triple helices by modified N-methylpyrrolo-dC nucleosides†
Abstract
3-Aminophenyl-modified analogues of the bicyclic nucleoside N-methyl-3H-pyrrolo[2,3-d]pyrimidin-2(7H)-one were synthesised and incorporated directly into triplex-forming

 Please wait while we load your content...
Please wait while we load your content...