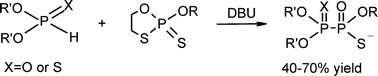
A new method for the formation of organohypophosphates containing a P–P bond under mild conditions, based on the DBU-assisted reaction of 2-alkoxy-2-thio-1,3,2-oxathiaphospholanes with O,O-dialkyl H-phosphonates or H-thiophosphonates, has been elaborated. The resulting triesters of P1-thio- and P1,P2-dithiohypophosphoric acids, respectively, having O-methyl or O-ethyl groups, can be selectively dealkylated to form the corresponding di- or monoesters. Appropriately protected 2′-deoxyguanosine-3′-O-(2-thio-1,3,2-oxathiaphospholane) was converted into the corresponding P1-thio- and P1,P2-dithiohypophosphate esters in a highly stereoselective manner (98%+ and 90%+, respectively).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...