N-alkylated oligoamide α-helical proteomimetics†
Abstract
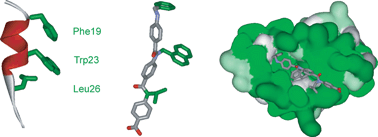
Generic approaches for the design and synthesis of small molecule inhibitors of protein–protein interactions (PPIs) represent a key objective in modern chemical biology. Within this context, the α-helix mediated PPIs have received considerable attention as targets for inhibition using small molecules, foldamers and proteomimetics. This manuscript describes a novel N-alkylated aromatic oligoamide proteomimetic scaffold and its solid-phase synthesis—the first time such an approach has been used for proteomimetics. The utility of these scaffolds as proteomimetics is exemplified through the identification of potent μM inhibitors of the p53–hDM2 helix mediated PPI—a key oncogenic target.

 Please wait while we load your content...
Please wait while we load your content...