A flexible approach for the asymmetric syntheses of hyacinthacines A2, A3 and structural confirmation of hyacinthacine A3†
Abstract
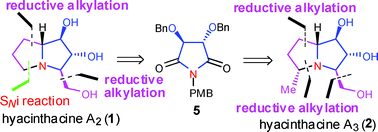
A concise and flexible approach for the asymmetric syntheses of polyhydroxylated pyrrolizidine alkaloids hyacinthacines A2 and A3 has been developed using iterative reductive

 Please wait while we load your content...
Please wait while we load your content...