Chemical and enzymatic stability of amino acid derived phosphoramidates of antiviral nucleoside 5′-monophosphates bearing a biodegradable protecting group†
Abstract
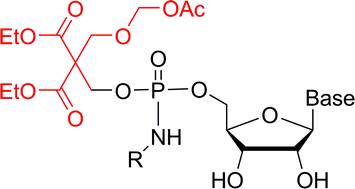
Ribavirin and 2′-O-methylcytidine 5′-phosphoramidates derived from L-alanine methyl ester bearing either an O-phenyl or a biodegradable O-[3-(acetyloxy)-2,2-bis(ethoxycarbonyl)propyl] or O-[3-(acetyloxymethoxy)-2,2-bis(ethoxycarbonyl)propyl] protecting group were prepared. The kinetics of the deprotection of these pro-drugs by porcine liver esterase and by a whole cell extract of human prostate carcinoma was studied by HPLC-ESI-MS/MS. The 3-(acetyloxymethoxy)-2,2-bis(ethoxycarbonyl)propyl and 3-(acetyloxy)-2,2-bis(ethoxycarbonyl)propyl groups were readily removed releasing the L-alanine methyl ester phosphoramidate nucleotide, the deprotection of the 3-(acetyloxymethoxy) derivative being approximately 20 times faster. The chemical stability of the 2′-O-methylcytidine pro-drugs was additionally determined over a pH range from 7.5 to 10.

 Please wait while we load your content...
Please wait while we load your content...