Efficient kinetic resolution of racemic 3-nitro-2H-chromene derivatives catalyzed by Takemoto's organocatalyst†
Abstract
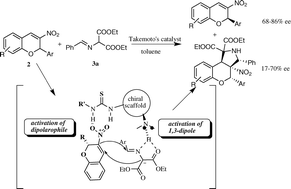
Efficient kinetic resolution of racemic 3-nitro-2H-chromenes by bifunctional thiourea afforded optically active (R)-3-nitro-2H-chromene derivatives with moderate to good enantioselectivities, which simultaneously gave the multifunctional 3,4-diphenyl-3a-nitrobenzopyrano-[3,4-c]-pyrrolidine-1,1-dicarboxylate derivatives with four vicinal chiral carbon centers.

 Please wait while we load your content...
Please wait while we load your content...