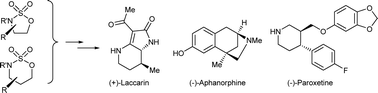
When combined with an appropriate nucleophilic component, 1,2- and 1,3-cyclic sulfamidates function as versatile precursors to a range of substituted and enantiopure heterocyclic classes. Functionalised enolates provide a direct entry to C-3 functionalised lactams, as exemplified by total syntheses of (−)-aphanorphine, (+)-laccarin and (−)-paroxetine. Heteroatom nucleophiles, such as thiol esters, amino esters and bromo phenols, provide concise access to a range of enantiomerically pure thiomorpholine, piperazine and benzofused heterocyclic scaffolds. The latter methodology enables a facile synthesis of the antibacteriocidal agent levofloxacin.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...