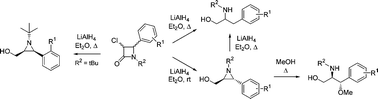
Stereoselective synthesis of trans- and cis-2-aryl-3-(hydroxymethyl)aziridines through transformation of 4-aryl-3-chloro-β-lactams and study of their ring opening
Abstract
trans- and cis-1-Alkyl-4-aryl-3-chloroazetidin-2-ones, prepared through cyclocondensation of

 Please wait while we load your content...
Please wait while we load your content...