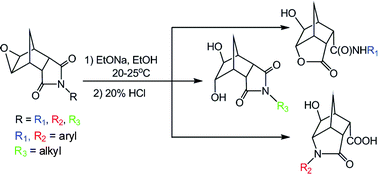
Combined experimental and theoretical studies have been carried out to investigate the transformations of the epoxyimides of norbornane into heterocyclic compounds. We established that interaction of the aryl-substituted epoxyimides of norbornane with sodium ethoxide results in the formation of new heterocyclic compounds in preparatively useful yields and with complete regioselectivity. The reactions of epoxyimides, containing aryl electron-donor substituents, result in the formation of endo-9-carbamoyl-exo-2-hydroxy-5-oxo-4-oxatricyclo[4.2.1.03,7]nonanes, while in the case of the absence of an aryl electron-donor group or the presence of aryl electron-withdrawing group in the epoxyimide, exo-2-hydroxy-5-oxo-4-azatricyclo[4.2.1.03,7]nonan-endo-9-carboxylic acids were obtained as products of the ethanolysis reaction. Unexpectedly, the ethanolysis of alkyl-substituted epoxyimides leads to dihydroxyimide formation as the major product. In order to understand the vital role of the imide substituent, a systematic theoretical DFT study at the PCM/B3LYP/6-31+G(d) level was carried out. We found that substituents at the nitrogen atom of epoxyimides exerted remarkable effects on the regioselectivity in the ethanolysis reaction, based on the solvent effects and intramolecular electronic interactions. Particularly, the preference for the formation of dihydroxyimides over heterocyclic systems for alkyl derivatives might be explained by kinetic stability of the formed acetal intermediate over the competitive epoxyamido acid intermediate. The above results provide a convenient and efficient method for predicting the structures of heterocyclic systems formed under basic ethanol conditions depending on the substituent on the nitrogen atom of the norbornane epoxyimides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...