Nucleophilic attack of 2-sulfinyl acrylates: A mild and general approach to sulfenic acid anions†
Abstract
An increasing number of reactions of sulfenic acid anions are being demonstrated in the literature. As such, mild, general and reliable means for the generation of sulfenates are due. In the current paper, an addition/elimination of 2-sulfinyl acrylates using various nucleophiles is demonstrated and evaluated as a protocol for
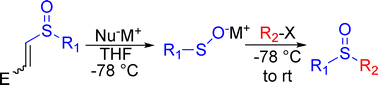

 Please wait while we load your content...
Please wait while we load your content...