Synthesis of novel synthetic intermediates from the reaction of benzimidazole and triazolecarbenes with ketenimines and their application in the construction of spiro-pyrroles†
Abstract
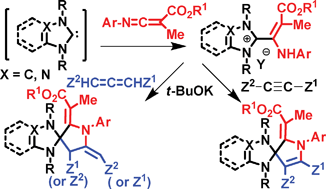
2-(2-Alkoxycarbonyl-1-arylamino-1-propenyl)benzimidazolium and 5-(2-alkoxycarbonyl-1-arylamino-1-propenyl)triazolium salts were synthesized in good yields from the reaction of

 Please wait while we load your content...
Please wait while we load your content...