Synthesis of fluorophosphonylated acyclic nucleotide analogues via copper(I)-catalyzed Huisgen 1-3 dipolar cycloaddition†
Abstract
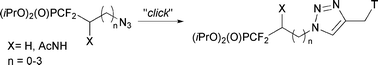
Preparation of several acyclonucleosides containing both a difluoromethylphosphonate group and a triazole moiety is described starting from a difluorophosphonosulfide. The key step of the synthesis involves a copper(I)-catalyzed Huisgen 1-3 dipolar

 Please wait while we load your content...
Please wait while we load your content...