Geometry-dependent divergence in the gold-catalyzed redox cascade cyclization of o-alkynylaryl ketoximes and nitrones leading to isoindoles†
Abstract
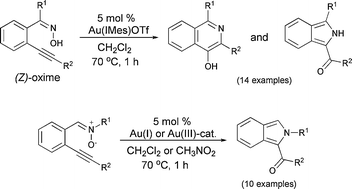
We report geometry-dependent cyclizations of o-alkynylaryl ketoximes and nitrones catalyzed by gold complexes. (E)-Ketoximes undergo N-attack to give isoquinoline-N-oxides. In sharp contrast, (Z)-ketoximes undergo unprecedented O-nucleophilic attack, followed by a redox cascade leading to a novel catalytic entry to

 Please wait while we load your content...
Please wait while we load your content...