N-Hexyl-4-aminobutyl glycosides for investigating structures and biological functions of carbohydrates†
Abstract
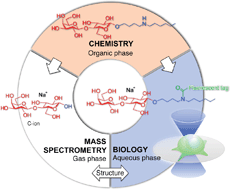
The potential applications of N-hexyl-4-aminobutyl glycosides in the mass spectrometric investigation of glycan structure and in the investigation of glycan functions were studied. Under

 Please wait while we load your content...
Please wait while we load your content...