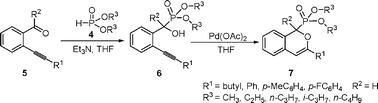
Efficient syntheses of phosphonylated isochromenes by regioselective 6-endo-dig addition to carbon-carbon triple bond catalyzed by Pd(OAc)2†‡
Abstract
Palladium(II)-catalyzed cycloisomerization of [(2-alkynylphenyl)hydroxymethyl]phosphonates 6 provides an efficient route to phosphonylated

 Please wait while we load your content...
Please wait while we load your content...