Full relative stereochemistry assignment and conformational analysis of 13,19-didesmethyl spirolide CviaNMR- and molecular modeling-based techniques. A step towards understanding spirolide’s mechanism of action†
Abstract
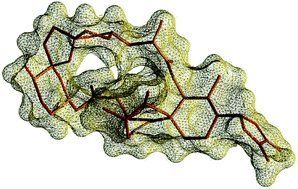
The relative stereochemistry of 13,19-didesmethyl spirolide C was determined through careful analysis of

 Please wait while we load your content...
Please wait while we load your content...