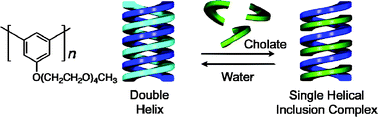
Double helix formation of poly(m-phenylene)s bearing achiral oligo(ethylene oxide) pendants and transformation into an excess of one-handed single helix through cholate binding in water†
Abstract
A water-soluble poly(m-phenylene) bearing an achiral oligo(ethylene oxide) chain at the 5-position was synthesized by the Ni(0)-mediated homo-coupling

 Please wait while we load your content...
Please wait while we load your content...