Synthesis of regio- and stereoselectively deuterium-labelled derivatives of l-glutamate semialdehyde for studies on carbapenem biosynthesis†
Abstract
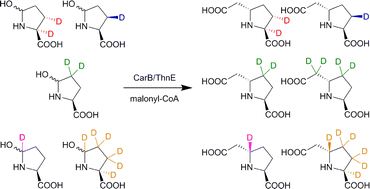
L-Glutamate semialdehyde (L-GSA) is an intermediate in biosynthetic pathways including those leading to the

 Please wait while we load your content...
Please wait while we load your content...