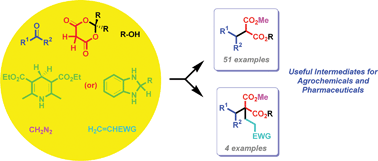
In this paper we describe new multi-catalysis cascade (MCC) reactions for the one-pot synthesis of highly functionalized non-symmetrical malonates. These metal-free reactions are either five-step (olefination/hydrogenation/alkylation/ketenization/esterification) or six-step (olefination/hydrogenation/alkylation/ketenization/esterification/alkylation), and employ aldehydes/ketones, Meldrum's acid, 1,4-dihydropyridine/o-phenylenediamine, diazomethane, alcohols and active ethylene/acetylenes, and involve iminium-, self-, self-, self- and base-catalysis, respectively. Many of the products have direct application in agricultural and pharmaceutical chemistry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...