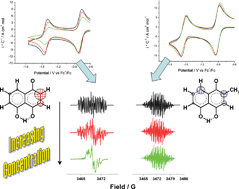
An electrochemical and spectroelectrochemical strategy is presented for evaluating reactivity differences in the semiquinone anions from naturally occurring quinones juglone (5-hydroxy-1,4-naphthoquinone) and plumbagin (2-methyl-5-hydroxy-1,4-naphthoquinone). By employing cyclic voltammetry and in situ spectroelectrochemical electron spin resonance measurements, it was found that while semiquinone species generated from plumbagin are stable radical anions in DMSO solution, the species generated from juglone are more reactive. These latter species are involved in a self-protonation process involving a slow rate of protonation (1.8–2.1 mol L−1) due to the mild acidity of the OH group at the C-5 position. This result is important when considering observed differences in biochemical reactivity for these quinones, particularly in cases where mediated cytotoxic action is provoked by these agents, as is discussed in this work.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...