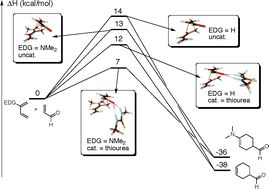
Density functional theory (DFT), using the hybrid functionals B3LYP and B2PLYP, has been employed to investigate the activation of the acrolein–butadiene Diels–Alder reaction, mediated by a thiourea catalyst. Effects due to electron-donating groups (EDGs) on the diene, as well as electron-withdrawing groups (EWGs) on the dienophile, have also been studied. Organic catalysts such as thioureas are known to lower the activation energy through hydrogen-bonding to the carbonyl oxygen, in a way that mimics the oxyanion holes of hydrolytic enzymes. EDGs and EWGs were found to further activate the reaction, and the catalyst showed a synergistic behavior towards the EDGs. Polar solvents were found to reduce the overall activation energy, but also the relative catalytic effect of the thiourea, in accordance with experimental studies. The substituent-mediated reactions displayed more asynchronous transition structures with lower activation energy, which led us to investigate the possibility of an alternative two-step, Michael-type route, similar to what has been found in macrophomate synthase. Although the concerted Diels–Alder route was found to be favored over the Michael route, the calculated activation energy difference is less than 1 kcal mol−1, which suggests that the two mechanisms compete, and could be responsible for the particular stereochemical outcome of an experiment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...