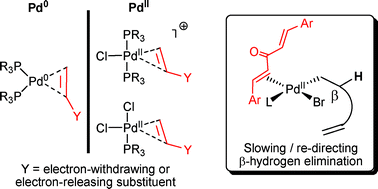
π-Acidic alkene (olefin) ligands positively influence Pd-catalysed cross-coupling processes, interacting with both palladium(0) and palladium(II) species, in some cases stabilising key catalytic intermediates. Rates of oxidative addition and reductive elimination are both affected. In certain cases, β-hydrogen elimination can be slowed down by π-acidic alkenes, which opens up new reaction pathways (e.g. interception of σ-alkylpalladium(II) species by appropriate nucleophiles). π-Acidic alkene ligands can act independently or in a synergistic fashion with another two-electron donor ligand (e.g. amine, phosphine or N-heterocyclic carbene). The purpose of this perspective article is to highlight the impressive results that can be obtained using π-acidic alkene ligands, with a particular focus on dibenzylidene acetone (dba) derivatives. Other types of alkene ligands, e.g. macrocyclic alkenes, are also discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...