Regioselective synthesis of functionalized 3,5-diketoesters and 2,4-diketosulfones by uncatalyzed condensation of 1-methoxy-1,3-bis(trimethylsilyloxy)-1,3-butadienes with α,β-unsaturated acid chlorides and sulfonyl chlorides†
Abstract
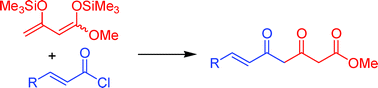
The reaction of 1-methoxy-1,3-bis(trimethylsilyloxy)-1,3-butadienes with α,β-unsaturated and functionalized acid chlorides afforded a variety of 3,5-diketoesters which are not readily available by other methods. The reaction of

 Please wait while we load your content...
Please wait while we load your content...