Colorimetric sensing of anions in aqueous solution using a charge neutral, cleft-like, amidothiourea receptor: tilting the balance between hydrogen bonding and deprotonation in anion recognition†‡
Abstract
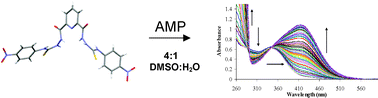
The design, synthesis and physical evaluation of 1, a visible colorimetric ‘naked eye’

 Please wait while we load your content...
Please wait while we load your content...