Nucleophilic reactivities of benzenesulfonyl-substituted carbanions†
Abstract
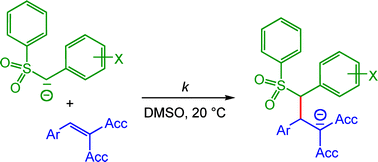
Kinetics of the reactions of four benzenesulfonyl-stabilized carbanions (1a–d)− with reference electrophiles (quinone methides 2 and diarylcarbenium ions 3) have been determined in dimethyl sulfoxide solution at 20 °C in order to derive the reactivity parameters N and s according to the linear free-energy relationship log k(20 °C) = s(N + E) (eqn (1)). The additions of (1a–d)− to ordinary Michael acceptors (e.g., benzylidene

 Please wait while we load your content...
Please wait while we load your content...