Enols and thioenols of substituted cyanomonothiocarbonylmalonamides: structures, enolizationvs. thioenolization, equilibria and conformations†‡§
Abstract
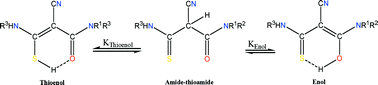
Condensation of organic isothiocyanates with cyanoacetamides gave 24 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OH)NR1R2 (11) or
C(OH)NR1R2 (11) or ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)CONR1R2 (13). The equilibrium constants (
C(CN)CONR1R2 (13). The equilibrium constants (

 Please wait while we load your content...
Please wait while we load your content...