Transient kinetic studies of protein hydrolyses by endo- and exo-proteases on a 27 MHz quartz-crystal microbalance
Abstract
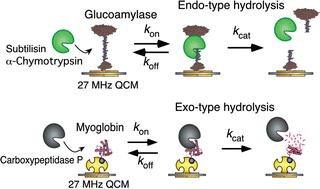
We have compared endo- and exo-type protease reactions and characterized the enzymatic reaction mechanisms by determining all kinetic parameters (kon, koff, kcat, Kd = koff/kon, and Km = (koff + kcat)/kon) by following the mass change of the formation and the decay of the enzyme–substrate (

 Please wait while we load your content...
Please wait while we load your content...