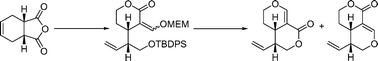
In the course of synthetic studies towards the development of diastereoselective routes to secoiridoid aglycones, cis-1,2,4,6-tetrahydrophthalic anhydride was transformed into the corresponding lactonecis-3a,4,7,7a-tetrahydro-3H-isobenzofuran-1-one, which served as a key precursor for a variety of chemoselective synthetic manipulations. Unsuccessful formylation of an ester intermediate resulted in a (E/Z) mixture of vinyl alcohols which were protected as acetates and as a single p-methoxybenzyl (PMB) ether (E) isomer. Dihydroxylation of the cyclohexene motif using OsO4 led to the unexpected deprotection of the PMB ether. On the other hand, successful formylation of a suitably silyl protected lactonised intermediate was achieved using tert-butoxybis(dimethylamino)methane, or Bredereck's reagent. Tetrabutylammonium fluoride (TBAF) deprotection of a methoxyethoxymethyl (MEM)-ether intermediate serendipitously afforded an approximately 1 : 1 mixture of pyrano-pyranones, which are products of a seldom encountered intramolecular Michael addition, using an oxygen donor, to the terminus of an α,β-unsaturated system, followed by β-elimination of the MEM moiety.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...