Reductive heterocyclizations via indium–iodine-promoted conversion of 2-nitroaryl imines or 2-nitroarenes to 2,3-diaryl-substituted indazoles
Abstract
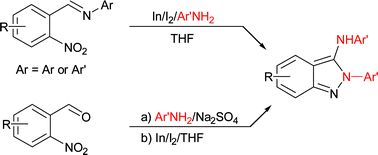
While N-(2-nitrobenzylidene)anilines produced mixtures of 2,1-benzisoxazoles and 3-anilino-2-aryl-2H-indazoles in the presence of indium and iodine in

 Please wait while we load your content...
Please wait while we load your content...