Preparation of new axially chiral bridged 2,2′-bipyridines and pyridyl monooxazolines (pymox). Evaluation in copper(i)-catalyzed enantioselective cyclopropanation†
Abstract
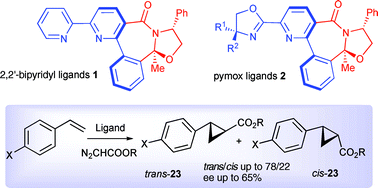
This work reports the synthesis of new axially chiral bridged 2,2′-bipyridines 1 and pyridylmonooxazolines (pymox) 2. The potential of these new axially chiral N,N-ligands was evaluated in asymmetric catalytic cyclopropanation of styrene derivatives 22a–c with diazoesters 21a,b. While 2,2′-bipyridines 1a–c afforded the corresponding cyclopropanes 23a–f in up to 65% ee, pymoxs 2a–e gave somewhat lower enantioselectivities (up to 53% ee). Both classes of ligands produced trans-cyclopropanes 23a–f as the major isomer, although with modest diasteroselectivities (56 : 44 to 78 : 22). A structure-stereoselectivity relationship study of ligands 1 and 2 identified the chiral biaryl axis as being mostly responsible for the enantioselective performances of these ligands.

 Please wait while we load your content...
Please wait while we load your content...