On the enantioselective olefinepoxidation by doubly bridged biphenyl azepine derivatives – mixed tropos/atropos chiral biaryls†
Abstract
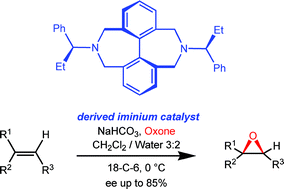
Diastereomeric doubly bridged biphenyl azepines, atropos at 20 °C and tropos at 80 °C, are precursors to effective iminium organocatalysts that are employed in the enantioselective epoxidation of prochiral

 Please wait while we load your content...
Please wait while we load your content...