Cyclic sulfamidates as versatile lactam precursors. An evaluation of synthetic strategies towards (−)-aphanorphine
Abstract
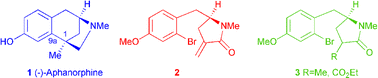
A full account of studies which led to the efficient asymmetric synthesis of (−)-aphanorphine 1 is reported. Two routes to the key cyclic

 Please wait while we load your content...
Please wait while we load your content...