Alkoxycarbonylation of aryl iodides using gaseous carbon monoxide and pre-pressurized reaction vessels in conjunction with microwave heating†
Abstract
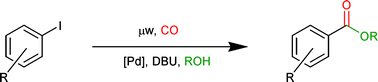
The microwave-promoted alkoxycarbonylation of aryl iodides using reaction vessels pre-pressurized with carbon monoxide is reported. Reactions are performed using 0.1 mol%

 Please wait while we load your content...
Please wait while we load your content...